Clinical Expertise & Experience
Having worked on a wide range of different clinical studies for over the past 2 decades, we provide experienced and dedicated staff with expertise in Phase I to IV studies, submission support (including ISS/ISE), and CDISC compliance across all major Therapeutic Areas.

Therapeutic Areas
With expertise spanning all research phases, we can support all major therapeutic areas including oncology, cardiovascular & metabolic and neurology from pre-clinical to post marketing. We collaboratively work with sponsors to turn challenges in rare diseases into opportunities by helping tackle small populations, slow recruitment, ethical issues, and endpoint selection. We blend deep therapeutic knowledge, and a client-focused approach to solve your dermatology trial challenges.
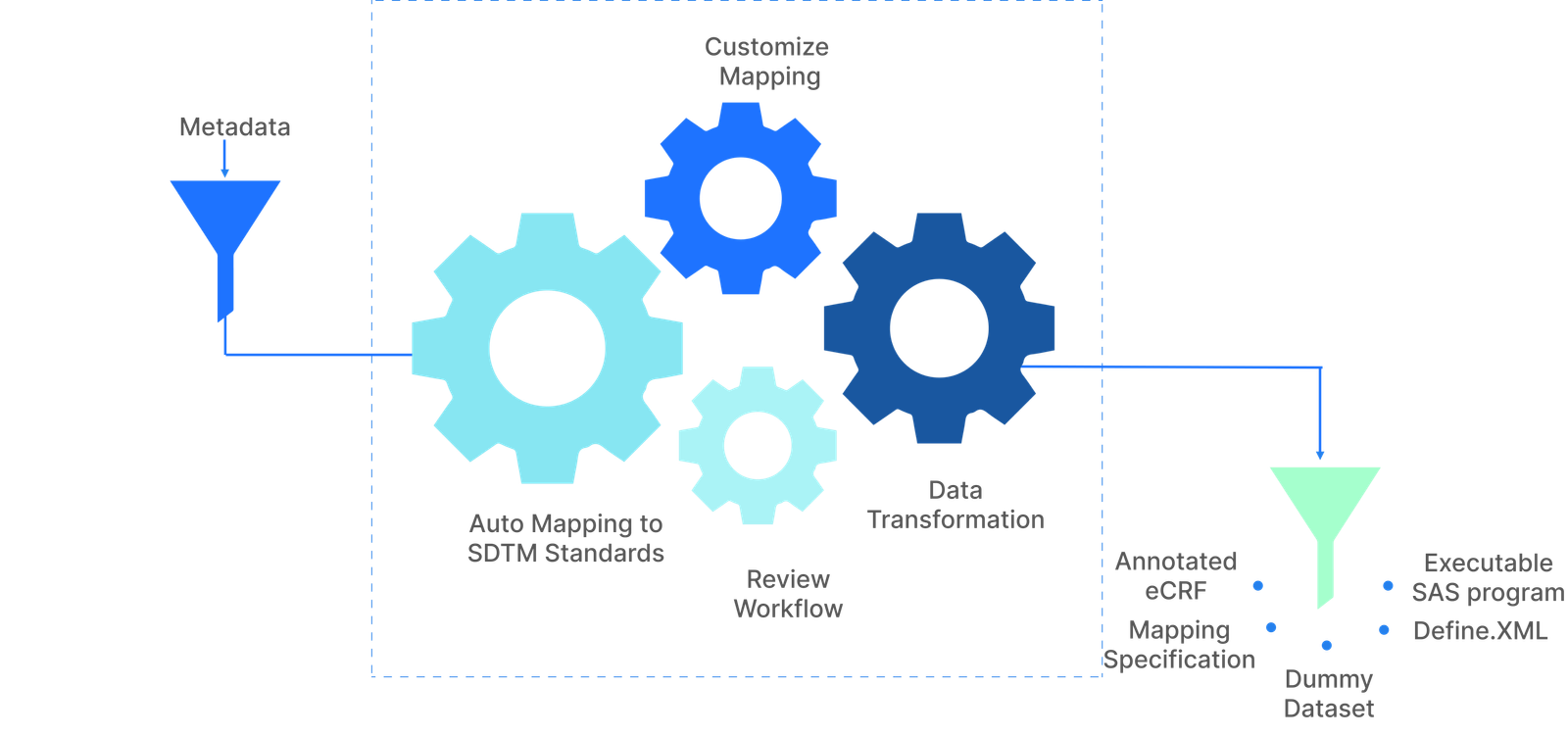
CDISC Services & Mapping
We have worked on large and small CDISC specific projects, some involving only consultancy and others involving mapping of multiple studies to CDISC standards. Our services include creation of CDISC SDTM domains from various customer defined data standards, creation of ADaM domains to support TFL output and maintain data traceability from CRF to CSR, and creation of the define.xml package, including reviewers’ guides, annotated CRFs and SAS V5 XPT files for regulatory submission to the FDA.
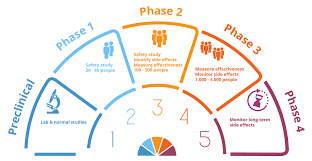
Clinic Trial Phases
We support studies in all phases but recognised the importance of phase I clinical trials and the special attention that they require. We have an excellent team of experts in this clinical phase and have built up the experience and knowledge to offer a complete package covering all aspects of phase I trial delivery. This covers the rapid delivery of core safety elements as well as advice and support to more novel objectives of specific trial designs.

Real World Evidence
We have extensive experience in the design and execution of real-world primary and secondary data collections to evaluate clinical, economic, epidemiological and patient-reported outcomes. We have the capacity and tools to design and undertake projects like secondary data use studies (chart reviews, existing database analysis) and primary data collection studies, ranging from simple cross sectional local surveys to large prospective multi-country non-interventional clinical studies.
